Press Releases
Biocon Biologics / Press Releases
Biocon Biologics to offer its Oncology Biosimilars through Cancer Access Partnership in over 30 countries

- Thu, 04-Feb-2021
- Posted by: Biocon Biologics
No Comments
Biocon Q3FY21 Revenue at ₹ 1,879 Crore, Up 7%; EBITDA at ₹427 Crore; Net Profit at ₹169 Crore; Biosimilars up 11% at ₹769 Crore; Research Services up 13% at ₹585 Crore; Generics down 3% at ₹561 Crore
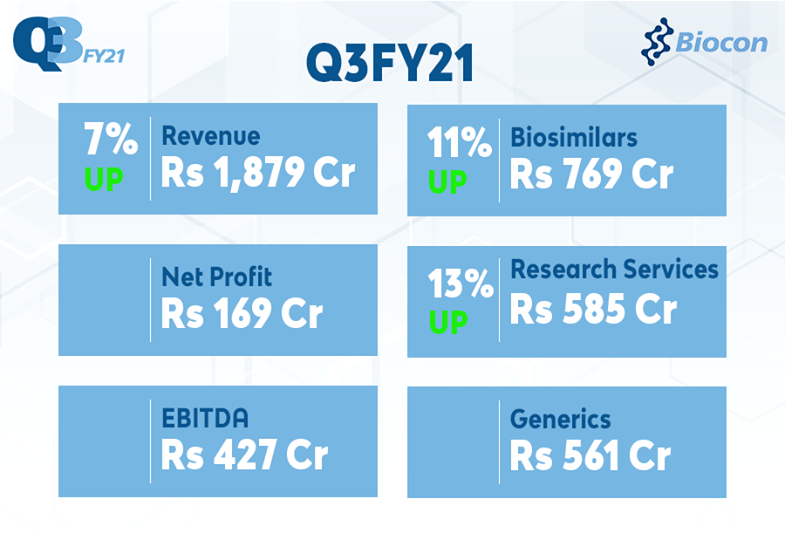
- Fri, 22-Jan-2021
- Posted by: Biocon Biologics
Professor Peter Piot Joins the Board of Biocon Biologics Limited as an Independent Director

- Thu, 21-Jan-2021
- Posted by: Biocon Biologics
Biocon Biologics Receives Rs 555 Cr (USD 75 Million) Capital Injection from ADQ

- Thu, 07-Jan-2021
- Posted by: Biocon Biologics
Biocon Biologics signs an MoU with CSSC in Tanzania for Mission 10 cents

- Tue, 15-Dec-2020
- Posted by: Biocon Biologics
Biocon Biologics Receives USD 150 Million Capital Injection from Goldman Sachs

- Sat, 07-Nov-2020
- Posted by: Biocon Biologics
Biocon Ranked Among Top 5 Biotech Employers Globally
- Fri, 30-Oct-2020
- Posted by: Biocon Biologics
Biocon Q2FY21 Revenue at Rs 1,760 Cr, Up 10%; EBITDA at Rs 407 Cr; Net Profit (before exceptional item & discontinuing operations) at Rs 174 Cr; Generics Up 8% at Rs 599 Cr; Biosimilars Up 11% at Rs 676 Cr; Research Services Up 12% at Rs 520 Cr.
- Fri, 23-Oct-2020
- Posted by: Biocon Biologics
Biocon Foundation Signs MoU with Bangalore Metro to Contribute Towards Building Metro Station in Hebbagodi

- Thu, 08-Oct-2020
- Posted by: Biocon Biologics
Biocon Biologics to Roll Out ‘Mission 10 cents’ in Philippines; Signs MoU with 2 Municipal Governments & reach52 to enable Affordable Access to Quality Insulins
- Wed, 30-Sep-2020
- Posted by: Biocon Biologics
 Brazil
Brazil Egypt
Egypt Europe
Europe Hong Kong
Hong Kong Malaysia
Malaysia Mexico
Mexico Morocco
Morocco Philippines
Philippines Saudi Arabia
Saudi Arabia South Africa
South Africa Taiwan
Taiwan Thailand
Thailand Tunisia
Tunisia Turkey
Turkey UAE
UAE USA
USA Vietnam
Vietnam Canada
Canada Global HQ
Global HQ
